Abstract
Introduction:
Axicabtagene ciloleucel (Axi-Cel), an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy demonstrated efficacy in patients with refractory large B cell lymphoma when conventional treatments failed. Cardiovascular side effects of CAR-T therapy that have been noticed so far include hypotension, left ventricular dysfunction, heart failure and cardiogenic shock in settings of CRS. We aimed to assess the cardiovascular side effects in a racially/ ethnically diverse patient population who underwent CAR-T cell therapy.
Methods:
This study included thirty-four consecutive adult patients who underwent treatment with CAR-T cell product Axi-Cel at an academic health system between 2018-2021. We performed detailed chart reviews and collected information related to the age, gender, hematological malignancy diagnosis, other medical comorbidities, therapeutic regimens, pretreatment cardiac risk factors, development of CRS with grading , pre and post treatment electrocardiograms ( EKG), transthoracic echocardiograms( TTE) , death, cause of death, and duration between administration of CAR-T products and death of the patients . We collected data pertaining to development of hypotension, EKG changes, arrhythmias, left ventricular systolic dysfunction, heart failure (HF), acute cardiac syndrome (ACS), troponin elevation, echocardiographic changes post CAR-T cell therapy, and follow up visits after 60 days to get information pertaining to development of hypotension, tachycardia or SOB, need for further cardiac work up, and cardiology referral.
Results:
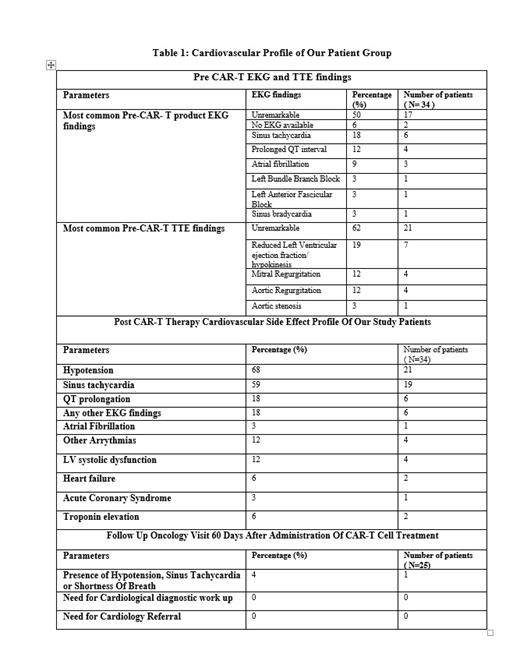
Mean age of our study participants was 65 years ranging between ages of 30 and 84 years with 38 % (13/34) female and 62% (21/34) male study participants. Study population was predominantly Hispanic, white and African American with percentages of 35% (12/34), 32% (11/34), 26 %(9/34) respectively followed by categories Asian 2.9% (1/34) and other at 2.9 %( 1/34). Sixty seven percent (22/37) patients had primary diffuse large B cell lymphoma (DLBCL) and 32% (11/34) had different primary malignancy with transformation into DLBCL. Thirty eight percent individuals had received autologous stem cell transplant. Sixty one percent (21/34) of our study participants developed cytokine release syndrome, with CRS grades 1-3 in 57% (12/29), 25% (08/21) and 4.7% (1/21) respectively. Thirty four percent (09/34) study participants died after cellular therapy. Septic shock and disease progression each were primary cause of death in 55 % (5/9) of patients, followed by respiratory failure in 22% (2/9) and ventricular fibrillation leading to cardiac arrest in 11% (1/9) of the patients. Mean duration of time between administration of therapy and death was 70 days. The cardiovascular effects noted immediately post CAR-T treatment and observations from follow up oncology visits are listed in Table 1. Troponin elevation was noticed in two study participants in settings of CRS, but one participant exhibited troponin elevation in absence of CRS. Seventeen percent of patients (6/34) developed left ventricular dysfunction after Axi-Cel. Most of them had concurrent CRS but one case of fatal heart failure occurred in absence of CRS. Three patients developed fatal arrhythmias post CAR-T therapy (1- Non sustained ventricular tachycardia in settings of CRS, 2- supraventricular tachycardia in settings of CRS, and 3- ventricular tachycardia needing defibrillation without CRS). Only one patient developed ACS in settings of CRS which lead to patient's demise.
Conclusions: In a real world, minority rich cohort, we observed that a significant number of our patients had preexisting cardiovascular findings including abnormal EKG (30% excluding sinus tachycardia) or abnormal echocardiographic findings (46%). Hypotension (68%) and sinus tachycardia (59%) were the most commonly observed cardiovascular toxicities. Although Axi-Cel was in general safe and well tolerated, we observed cardiovascular side effects associated with and independent of CRS. Notably, six patients (17%) developed left ventricular dysfunction including one fatality which was independent of CRS. There was only one fatal coronary syndrome and two cases of troponin elevation in our series. Our study stresses the importance of a thorough cardio-oncology evaluation before proceeding with cellular therapies as well as involved follow up during and after hospitalization.
Gritsman: iOnctura: Research Funding. Shastri: Kymera Therapeutics: Research Funding; Onclive: Honoraria; Guidepoint: Consultancy; GLC: Consultancy. Verma: BMS: Research Funding; GSK: Research Funding; Incyte: Research Funding; Medpacto: Research Funding; Curis: Research Funding; Eli Lilly: Research Funding; Stelexis: Consultancy, Current equity holder in publicly-traded company; Novartis: Consultancy; Acceleron: Consultancy; Celgene: Consultancy; Stelexis: Current equity holder in publicly-traded company; Throws Exception: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal